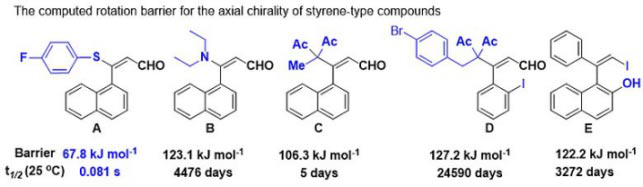
Construction methods include asymmetric oxidation/cross-coupling of two aromatic nucleus segments, chiral construction of aromatic nucleus, and kinetic resolution/desymmetrization of biaryl compounds, etc. As an important class of compounds, styrene has a wide range of applications in chemical synthesis and asymmetric catalysis, but there are few related studies on chiral styrene derivatives, a new class of chiral compounds.
Alhough the concept of Axial chirality has been mentioned in the study of chiral memory by people like Kawabata, follow-up reports on the synthesis and application of chiral styrene are rarely seen. That is mainly due to the fact that the rotation energy barrier of C-C bond axis in styrene is relatively low and it is rather difficult to control the stereoselectivity of the reaction.
After determining the existence of axial chirality of these compounds, they chose 2-substituted phenylacetyl aldehyde as the substrate, then let the compounds react with two nucleophilic reagents, 1,3- Curdione andMalononitrile, under the catalysis of chiral secondary amine to construct Axial chiral Styrene aldehyde. In this way, they, with high yield, cis trans selectivity and enantioselectivity, synthesised this new kind of Axially Chiral Compounds of styrene skeleton which can be scaled up to gram scale,achieving the asymmetric control of such axially chiral compounds for the first time.
Subsequently, the authors studied the suitability of the substrate, which showed that the reaction had good substrate universality. Finally, they performed a series of transformations on the substrate and successfully expanded the synthesis and application of axisymmetric styrene aldehydes (see the figure below). The report on the study was published on Nature Communications.
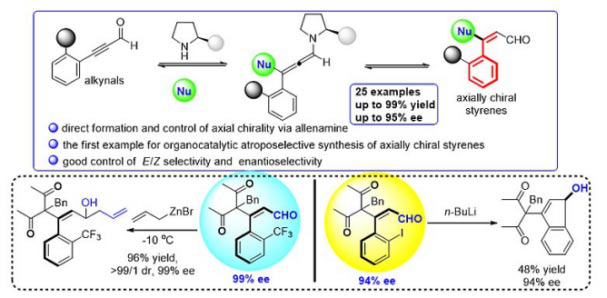
The simple styrene structure is an important raw material for chemical synthesis. Due to the presence of the double bonds in it, it can be used to carry out many subsequent chemical transformations to obtain key intermediates, and also can serve as chiral ligand to catalyze asymmetric reactions. Therefore, it is necessary to develop asymmetric catalytic reactions to synthesize axially chiral styrene derivatives with diverse structure. Given the importance of axial chiral compounds with a styrene skeleton, we can predict that the development of such strategies will lead to more designs relating to Axially Chiral reactions.


